Nestlé Heath Science has developed a range of nutritional solutions to help meet the needs of infants and children with food allergies and intolerances. Althéra®, Alfaré®, and Alfamino® provide adequate nutrients to support normal infant growth and development.1-4

Following diagnosis, CMPA is managed through the complete removal of cow’s milk proteins (CMPs) from the infant’s diet. CMPA can also occur among exclusively breastfed infants. When a breastfed infant is diagnosed with CMPA, the mother must exclude all CMP from her diet.
Mothers should be encouraged to continue breastfeeding, but in case this is not an option, specialty formulas are available for infants and young children with CMPA to reduce or completely remove the allergenic potential. These formulas contain the adequate nutrients to support healthy infant growth and development. There are two different types of formulas for the management of CMPA:
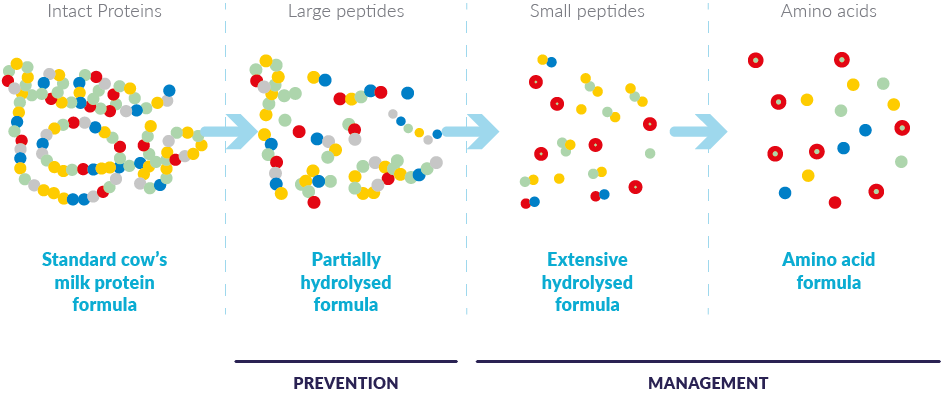
Extensively hydrolyzed formulas,* or “eHFs,” in which CMPs have been extensively hydrolyzed into peptides. As a result, they are less allergenic than whole milk formulas and are very well tolerated by most children with cow’s milk or soy protein allergies.
Amino acid-based formulas (AAFs) contain free amino acids, which are non-allergenic. They are recommended when a baby does not tolerate an extensively hydrolyzed formula or as first-line management when the baby has extremely severe or life-threatening symptoms (anaphylaxis or immediate type reactions).1
*Note: Partially hydrolyzed formulas (pHFs) are also sometimes referred to as hypoallergenic; however, pHFs are not intended for dietary management of diagnosed CMPA.
OUR RANGE OF TAILOR-MADE NUTRITIONAL SOLUTIONS
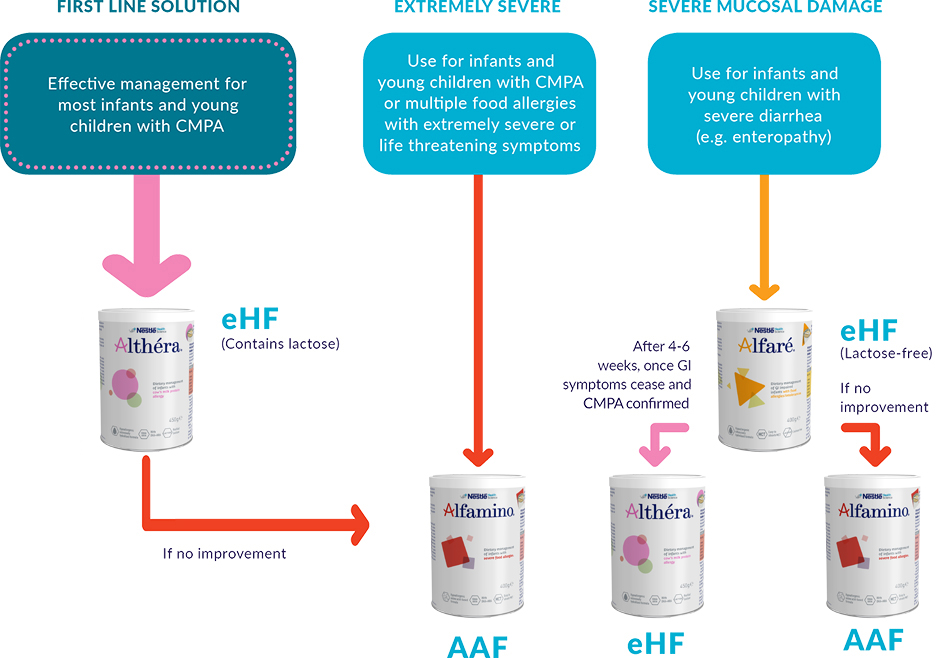
Mothers should be encouraged to continue breastfeeding, but in case this is not an option, Nestlé Health Science has developed a decision tree to support healthcare professionals in the dietary management of food allergies and intolerances. Following diagnosis, this decision tree will help you to consider the possible options for your patients based on their symptoms and will help you in selecting the right solution at the right time.
- Vandenplas Y, et al. Safety and adequacy of an optimized formula for pediatric patients with cow’s milk-sensitive enteropathy. Minerva Pediatr. 2010;62(4)339–45.
- Vandenplas Y, et al. Safety and adequacy of a semi-elemental formula for children with gastro-intestinal disease. Amino Acids. 2010;38(3):909–14.
- Niggemann B, et al. Safety and efficacy of a new extensively hydrolyzed formula for infants with cow’s milk protein allergy. Pediatr Allergy Immunol. 2008;19(4):348–54.
- Nowak-Wegrzyn A, et al. Evaluation of hypoallergenicity of a new, amino acid-based formula. Clin Pediatr (Phila). 2015;54(3) 264–72.
- Rapp M, et al. Characterization of an extensively hydrolyzed whey infant formula with a low bitterness. Clin Transl Allergy. 2013; 3(Suppl 3): P132.
- Schappi M, et al. Omega 3PUFA enriched semi-elemental diet for protracted diarrhoea (abstract). ESPGHAN. 2006. Abstract PG3-14.
- Milla P, et al. A new semi-elemental diet for small intestinal inflammatory disease (abstract). ESPGHAN. 2004. Abstract PO583.
- Infant nutrition and feeding. A guide for use in the WIC and CSF programs. March 2009:14.
- Francavilla R, et al. Effect of lactose on gut microbiota and metabolome of infants with cow’s milk allergy. Pediatr Allergy Immunol. 2012;23(5):420–7.



